Was the Catalytic Converter the Key to Cleaning Up Air Pollution?
Unlocking the Key to Cleaner Air: How Catalytic Converters Changed Pollution
Source www.carboncleaningmachine.com
When Was the Catalytic Converter Invented?
The catalytic converter is one of the most important inventions in the field of automobile engineering as it helps reduce the harmful emissions that vehicles release into the environment. This innovative device has been playing a significant role in maintaining the air quality by filtering harmful pollutants since its first development. In this article, we will provide a detailed overview of when the catalytic converter was invented and how it has evolved over time to meet the changing environmental regulations.
The Need for Emissions Control
The growing concerns regarding air pollution from combustion engines led to the need for emissions control devices such as the catalytic converter. The catalytic converter was designed to control the emission of harmful pollutants, such as carbon monoxide, hydrocarbons, and nitrogen oxides, from the exhaust system of vehicles. The use of catalytic converters in vehicles helps to prevent air pollution and also plays an important role in improving public health.
Early Developments
The first patents related to catalytic converters date back to the 1950s, but it wasn't until the 1970s that practical developments began. Eugene Houdry, a French mechanical engineer, is credited with developing the first successful catalytic converter in the early 1950s. By the end of the decade, a few automobile manufacturers began using Houdry's design to reduce the emissions from their vehicles.
However, it was in the 1970s that automobile manufacturers began using catalytic converters on a large scale. This was primarily due to the increasing awareness of the harmful effects of vehicle emissions on the environment and public health. In 1975, the United States Environmental Protection Agency (EPA) established regulations that mandated the use of catalytic converters in all new vehicles to reduce the emission of harmful pollutants.
The Modern Catalytic Converter
By the 1980s, the catalytic converter became a standard feature in most gasoline-powered vehicles, and stricter regulations led to improvements in its efficiency. As a result, the modern catalytic converter is much more effective in converting harmful pollutants into less harmful substances. Over the years, the catalytic converter has undergone various design changes to make it more efficient and durable.
Today, the catalytic converter is a mandatory component of all gasoline and diesel-powered vehicles in many countries worldwide. However, some countries still have cars on the road that do not have a catalytic converter. These vehicles are considered highly polluting and contribute significantly to the air pollution levels, making it essential to eliminate them from the roads.
Conclusion
In conclusion, the catalytic converter was invented to control emissions from vehicles and reduce their environmental impact. The use of this simple yet innovative technology has led to significant improvements in the air quality and public health. Over time, the catalytic converter has undergone numerous design changes to become more efficient and meet the stringent environmental regulations. Today, it is a standard feature in most vehicles across the globe and plays a vital role in protecting the environment and public health.
How Does a Catalytic Converter Work?
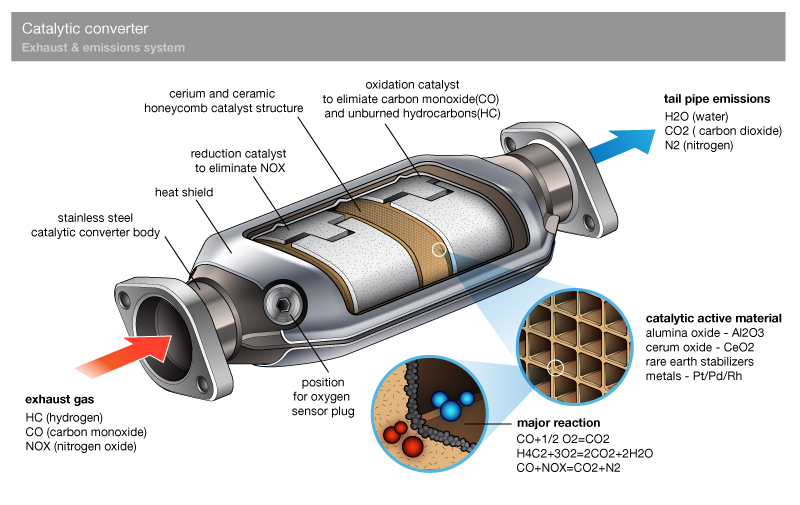
Catalytic converters are designed to reduce the amount of pollutants that are released into the atmosphere. Harmful pollutants in exhaust gases are converted into less harmful substances through chemical reactions. The converter works by creating an environment that allows the pollutants to react with each other, breaking them down into less harmful components.
The Chemical Reactions
The chemical reactions that take place in the catalytic converter rely on the presence of precious metals, such as platinum, palladium, and rhodium. As the exhaust gases pass through the converter, they come into contact with the metals, which act as a catalyst for the reactions. The metals promote a series of chemical reactions that break down the pollutants into less harmful substances.
When nitrogen oxides and carbon monoxide come into contact with the catalyst, they react to form nitrogen gas, carbon dioxide, and water vapor. Hydrocarbons also undergo a series of chemical reactions, breaking down into carbon dioxide and water vapor.
The Three-Way Catalytic Converter
The most common type of catalytic converter is the three-way catalytic converter. It is named this way because it is capable of reducing three harmful pollutants, nitrogen oxides, carbon monoxide, and hydrocarbons. The three-way converter is a highly sophisticated system that relies on precise engineering and accurate control systems.
When the engine is running, exhaust gases are released into the converter. The three-way system is designed to have three zones: an oxidizing zone, a reduction zone, and a storage zone. The oxidizing zone is where the nitrogen oxide is converted into nitrogen and oxygen. The reduction zone is where the carbon monoxide and hydrocarbons are converted into carbon dioxide and water vapor. Finally, the storage zone is where the oxygen is stored, waiting to be used in the next reaction.
The Role of Oxygen Sensors
Oxygen sensors play a crucial role in ensuring that the catalytic converter is working effectively. They help regulate the air-to-fuel ratio in the engine, which is crucial for the converter to work efficiently. If the air-to-fuel ratio is too rich or too lean, it can cause the converter to become overloaded or overheated, leading to premature failure.
Modern cars are equipped with sophisticated oxygen sensors that are designed to provide accurate readings of the air-to-fuel ratio. The sensors use a voltage signal to indicate whether the engine is running too rich or too lean. If the signal indicates a problem, the engine's electronic control module can adjust the air-to-fuel ratio, ensuring that the converter is working effectively.
In summary, the catalytic converter is an essential component of modern cars that helps reduce harmful pollutants that are released into the atmosphere. The converter relies on chemical reactions to break down pollutants into less harmful components, and the three-way catalytic converter is the most common type, capable of reducing nitrogen oxides, carbon monoxide, and hydrocarbons. Oxygen sensors play a critical role in ensuring that the converter is working effectively, regulating the air-to-fuel ratio in the engine.
Catalytic Converter Materials
The catalytic converter is an important component in modern vehicles that helps reduce harmful emissions. Invented in the 1970s, it played a vital role in the fight against pollution and smog. The catalytic converter works by converting harmful gases and pollutants from the exhaust into less harmful emissions. It functions on the principles of catalysis, which involves the use of a catalyst, a substrate, and a canister.
The Catalyst
The catalyst plays a crucial role in the functioning of the catalytic converter. It primarily works by accelerating the reaction between two or more substances, which usually leads to the breaking down of chemical bonds and the formation of new ones. In the catalytic converter, the catalyst helps convert the harmful gases and pollutants from the exhaust into less harmful emissions.
Typically, the catalyst is usually made from precious metals like platinum, palladium, and rhodium. These metals are chosen for their unique ability to catalyze reactions at high temperatures. Platinum is the most commonly used catalyst, followed by palladium and rhodium.
The Substrate
The substrate is a crucial component in the catalytic converter as it acts as a support for the catalyst and provides a large surface area for the exhaust gases to pass through. The substrate needs to have high heat resistance and structural strength to withstand the high temperatures and vibrations in the exhaust system.
Typically, two materials are used as substrates in catalytic converters: metallic and ceramic. Metallic substrates are made from stainless steel and are widely used in heavy-duty vehicles. Ceramic substrates, on the other hand, are composed of aluminum oxide and are preferred in most light-duty vehicles due to their higher efficiency.
The Canister
The canister is an essential component in a catalytic converter, and it encloses the substrate and the catalyst. It is designed to resist high-temperature corrosion, which is a common problem in most exhaust systems, and it also helps distribute the exhaust gases evenly over the substrate. Canisters are usually made from stainless steel or other corrosion-resistant metals like titanium.
The canister design needs to be carefully thought out to ensure that the catalytic converter performs optimally. It should be able to control exhaust gas flow, minimize backpressure, and withstand high temperatures for extended periods.
In conclusion, the catalytic converter is an essential component in modern vehicles, and it plays a crucial role in reducing harmful emissions. The effectiveness of the catalytic converter relies heavily on the quality of the components used. The catalyst, substrate, and canister are all essential components that work together to make the catalytic converter function efficiently. Understanding the materials used in the catalytic converter can help appreciate the important role it plays in protecting our environment.
When Was the Catalytic Converter Invented?
The catalytic converter is an essential part of modern vehicles, reducing harmful emissions and protecting the environment. However, it wasn't always a standard component of cars. Let's take a closer look at the history of the catalytic converter.
The Development of the Catalytic Converter
The catalytic converter was invented in response to the growing environmental concerns in the 1950s and 1960s. At that time, the emissions from vehicles and industrial sources were causing significant air pollution and health problems.
The first catalytic converter was developed in the mid-1950s by French engineer Eugene Houdry. He had previously worked on the development of fluid catalytic cracking, a process used to refine petroleum. Houdry realized that the same technology could be applied to the exhaust emissions from vehicles.
In 1975, the United States Environmental Protection Agency (EPA) made the catalytic converter mandatory for all new cars sold in the country, making it a standard component of modern vehicles.
How the Catalytic Converter Works
The catalytic converter is a device that converts harmful pollutants in vehicle exhaust into less harmful compounds. Inside the converter, a ceramic or metallic substrate is coated with a catalyst material, usually a combination of platinum, palladium, and rhodium.
When the exhaust gases pass through the converter, the catalyst materials cause a chemical reaction that breaks down the pollutants and converts them into water vapor, carbon dioxide, and nitrogen. The result is a significant reduction in harmful emissions, including nitrogen oxides, carbon monoxide, and hydrocarbons.
Catalytic Converter Maintenance
Proper maintenance of your vehicle's catalytic converter is essential to ensure it continues to function effectively and reduce harmful emissions. Here are some tips:
Location and Access
Catalytic converters are located in the exhaust system of vehicles and can be accessed from underneath the car or the hood.
Signs of a Worn Out Converter
A damaged or worn out catalytic converter can cause various symptoms like reduced engine performance, increased emissions, and strange noises.
Replacing a Catalytic Converter
Replacing a catalytic converter can be expensive, and it's important to choose the right type and size for your vehicle and to have it installed by a certified mechanic.
Regular maintenance of your vehicle's catalytic converter is essential for proper functioning and reduced emissions. Be sure to have your car inspected by a reliable mechanic to identify any problems early and extend the life of your catalytic converter. Together, we can help protect the environment and reduce air pollution.
The Future of Catalytic Converters
New Technologies
As the world continues to shift towards cleaner energy sources, the development of new technologies for catalytic converters has become increasingly important. Researchers are exploring new materials and designs that could improve the efficiency, durability, and cost-effectiveness of these devices.
One approach involves using nanotechnology to develop more efficient catalysts with larger surface areas, which could increase their performance while reducing the amount of precious metals needed. Another avenue of research is focused on developing new catalytic converter designs that better integrate with modern automobile engines, improving their function and reducing emissions.
Alternative Solutions
The growing popularity of electric and hybrid vehicles has led some to question the continued relevance of catalytic converters. These vehicles produce much less emissions than traditional combustion engines, and some experts believe that they may eventually make catalytic converters obsolete.
While this may be true for vehicles that rely entirely on electric power, hybrid vehicles still rely on traditional engines for at least part of their operation. This means that these vehicles, and the vast majority of cars and trucks on the road today, will continue to require catalytic converters for the foreseeable future.
Environmental Impact
Catalytic converters have had a significant positive impact on air quality, reducing the amount of harmful pollutants released into the atmosphere by vehicles. However, their production requires the mining and refining of precious metals, which can have negative environmental consequences.
Some companies are exploring ways to minimize the environmental impact of catalytic converter production, such as using recycled metals and implementing more sustainable mining practices. Additionally, advancements in nanotechnology and catalyst design may lead to more efficient catalytic converters that require less precious metals to produce, reducing their environmental impact.
In conclusion, while the catalytic converter may not be the only solution for reducing vehicle emissions in the future, it will remain an important part of our efforts to combat air pollution. Continued research into new materials and designs, as well as more sustainable production methods, will help ensure that these devices remain effective and feasible for years to come.